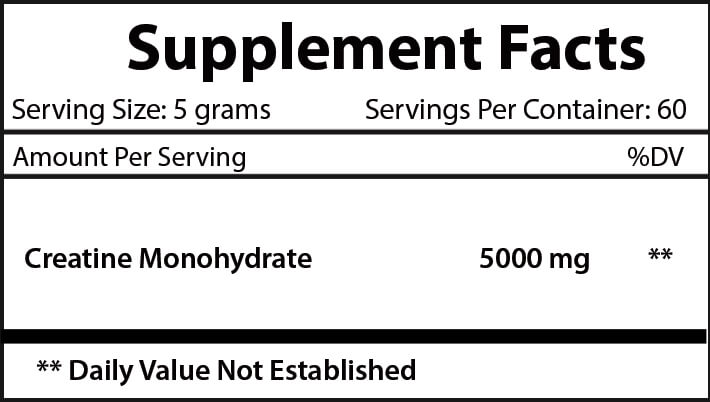
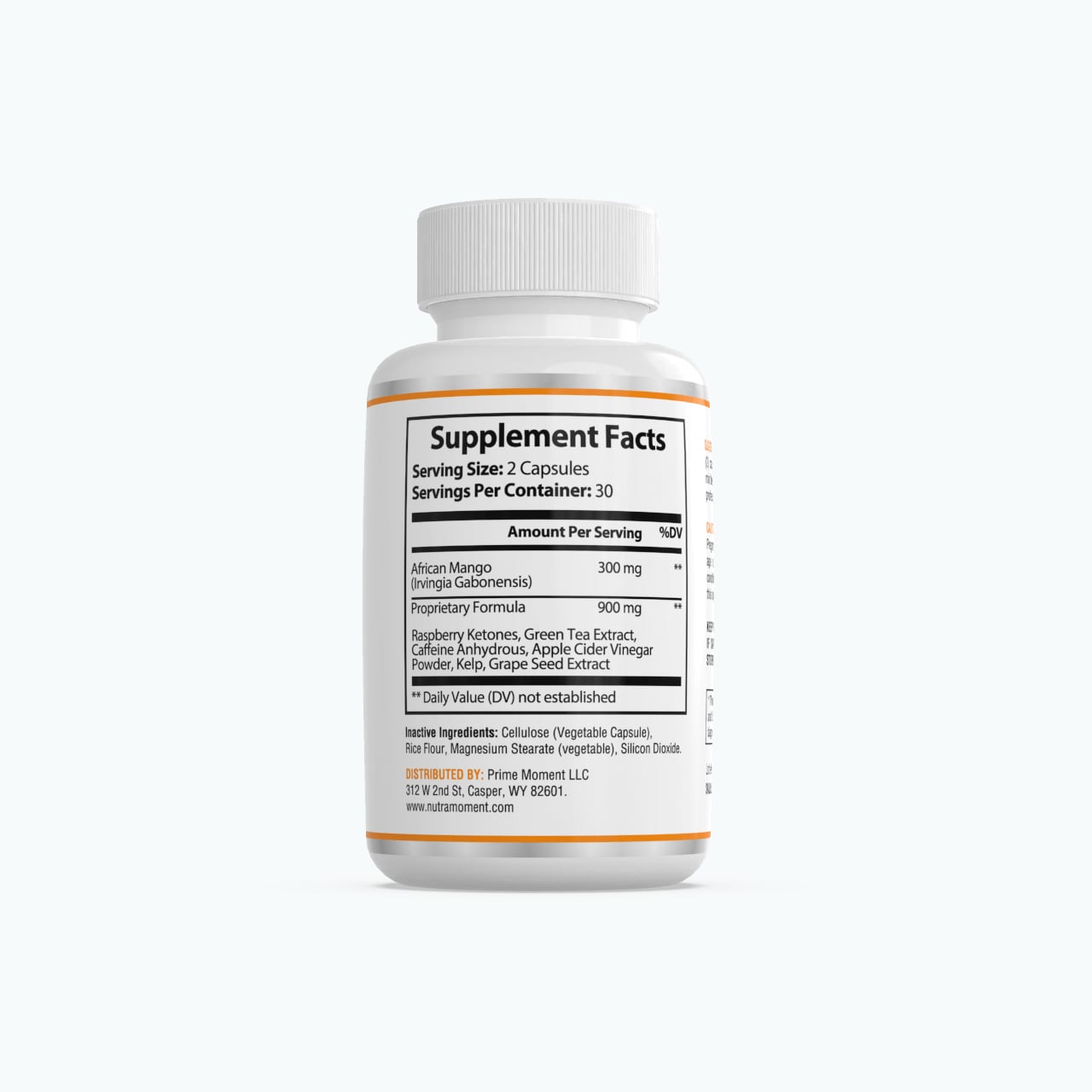
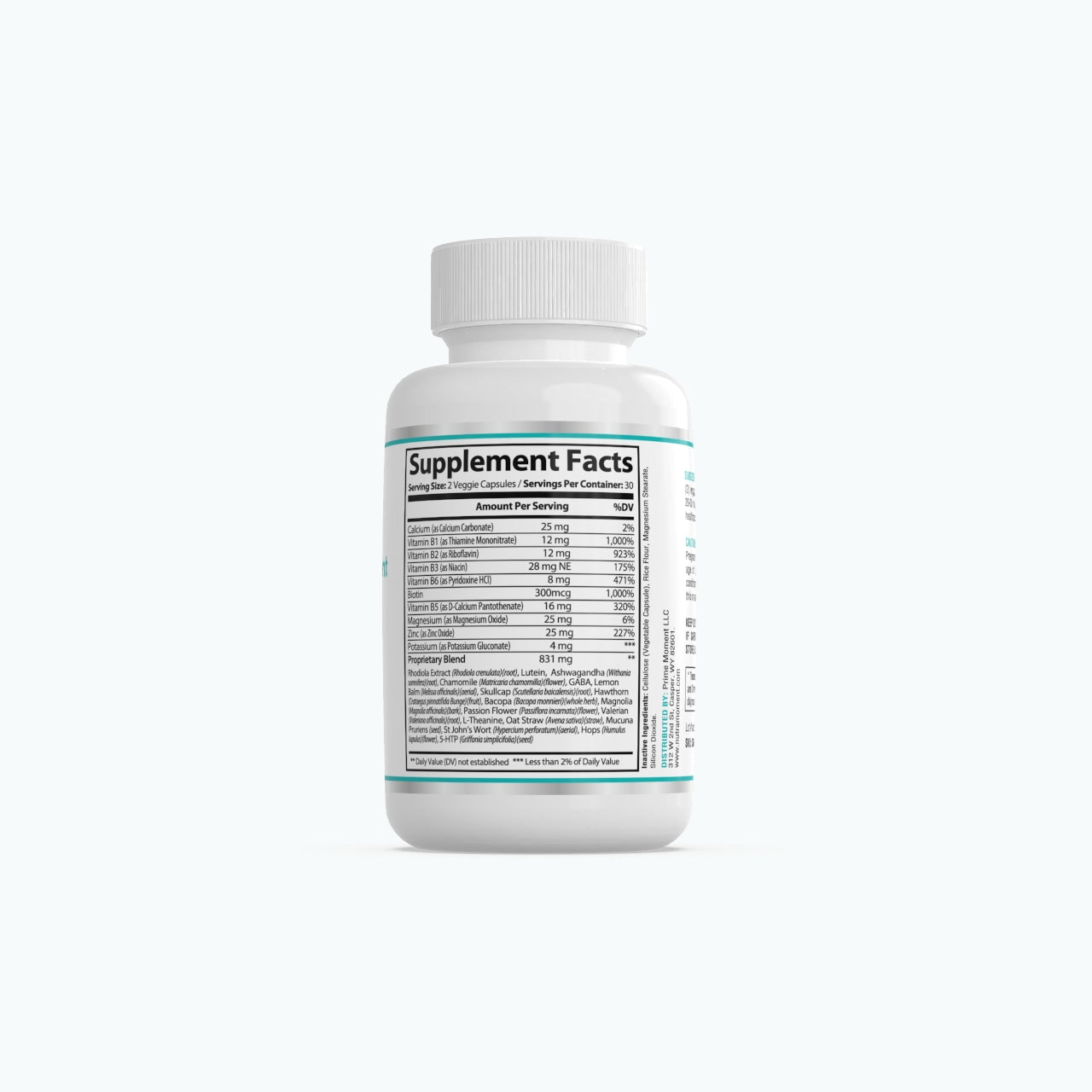
FDA Disclosure
The statements made regarding these products have not been evaluated by the Food and Drug Administration. The efficacy of these products has not been confirmed by FDA-approved research. Actimore assumes no responsibility for the improper use of these products. These products are not intended to diagnose, treat, cure or prevent any disease. All information presented here is not meant as a substitute for or alternative to information from health care practitioners. Please consult your health care professional about potential interactions or other possible complications before using any product. The Federal Food, Drug, and Cosmetic Act require this notice. Our website showcases our entire product catalog. Our cannabidiol products are available for purchase at actimore.com Cannabidiol (CBD) is a natural component of hemp oil.
THC Disclaimer
Most workplace drug screens and tests target delta9-tetrahydrocannabinol (THC) and do not detect the presence of other legal natural hemp-based constituents. However, studies have shown that eating hemp foods and oils can cause confirmed positive results when screening urine and blood specimens. Accordingly, if you are subject to any form of drug testing or screening, we recommend (as does the United States Armed Services ) that you do not ingest our products. Prior to consuming these products consult with your healthcare practitioner, drug screening /screening/testing company, or employer. Actimore works with suppliers who guarantee less than or equal to 0.3% THC content. With these trace amounts of THC, it is highly unlikely that you will fail a drug test however, Actimore does not take responsibility in the instance a customer fails a drug test while taking these products.